Cement is one of the most widely used materials in the construction industry. It is the primary ingredient in concrete, which forms the backbone of modern infrastructure. The manufacture of cement involves several steps, each contributing to the final product’s strength, durability, and versatility. This article outlines the history, materials, and step-by-step manufacturing process of Portland cement, with a focus on the chemical reactions involved in each stage.
Introduction
Portland cement, named for its resemblance to a particular stone quarried on the Isle of Portland in England, is a fine, dry powder that, when mixed with water, hardens into a strong, durable material. The process of making cement has evolved over centuries, with its roots stretching back to the Roman Empire. However, the modern version of Portland cement, which is used extensively today, was developed in 1824 by British stone mason Joseph Aspdin. He first created the cement by heating a mixture of limestone and clay in his kitchen, then grinding it into a fine powder. His invention revolutionized construction, and by 1828, Portland cement was being used in the construction of the Thames River Tunnel.
Materials and Manufacturing Process of Portland Cement
The primary raw materials used in Portland cement production are calcium, silicon, iron, and aluminum. These elements are derived from limestone, clay, and other natural resources. Depending on the availability of raw materials, they may be used in different forms. The right proportions of these materials are crucial in ensuring the desired chemical composition of the final product.
The manufacturing process of Portland cement involves a series of steps that include the mixing of raw materials, burning of the mix in a rotary kiln, grinding of the resulting clinkers, and packaging of the finished product. The methods used in each of these steps can vary, with two main approaches: the dry process and the wet process.
Manufacturing Process of Portland Cement
1. Mixing of Raw Materials
The first step in cement manufacturing is the mixing of the raw materials. The key materials required for Portland cement production include:
- Limestone (Calcium carbonate): The primary source of calcium.
- Clay or Shale (Silicon, Aluminum, and Iron): Sources of silicon, aluminum, and iron.
- Other materials: Occasionally, other minerals may be added to adjust the composition.
The mixing of these materials can be done using two methods: Dry Process and Wet Process.
a) Dry Process
In the dry process, both the calcareous (limestone) and argillaceous (clay) raw materials are crushed separately in gyratory crushers to reduce them to pieces of 2-5 cm in size. These crushed materials are then finely ground into powder in ball or tube mills. After grinding, the powders are stored in hoppers and then blended in specific proportions. This dry raw mix is stored in silos until it is ready for the next step in the process—being fed into the rotary kiln.
b) Wet Process
In the wet process, the raw materials are crushed into a fine powder and stored in silos. The clay is washed to remove organic matter and impurities. The powdered limestone and water-washed clay are then mixed in slurry form in grinding mills. This slurry, which contains about 38-40% water, is stored in tanks before being sent to the rotary kiln. The wet process typically leads to a higher quality product but requires more energy and time compared to the dry process.
2. Comparison of Dry and Wet Processes
| Criteria | Dry Process | Wet Process |
|---|---|---|
| Hardness of Raw Material | Quite hard | Any type of raw material |
| Fuel Consumption | Low | High |
| Time of Process | Shorter | Longer |
| Quality | Inferior | Superior |
| Cost of Production | Higher | Lower |
| Physical State of Raw Mix | Solid | Slurry (liquid) |
3. Burning of Raw Materials
Once the raw materials are mixed, they are fed into a rotary kiln. The rotary kiln is a large cylindrical tube, typically 90 to 120 meters in length and 2.5 to 3 meters in diameter, made from steel and lined with refractory bricks to withstand high temperatures. The kiln is inclined slightly to ensure that the materials move down the length of the furnace as it rotates.
The burning process in the kiln is divided into three distinct zones:
- Drying Zone: In the upper part of the kiln, the temperature is around 400°C. Here, any moisture in the raw materials evaporates.
- Calcination Zone: In the middle part of the kiln, at around 1000°C, limestone (calcium carbonate) decomposes into calcium oxide (lime) and carbon dioxide (CO2): CaCO3→CaO+CO2
- Clinkering Zone: In the lower part of the kiln, temperatures reach 1500-1700°C. In this region, lime reacts with silicon and aluminum compounds in the raw material to form calcium silicates and aluminates, which fuse to form hard, small stones known as clinkers. The chemical reactions that occur include:
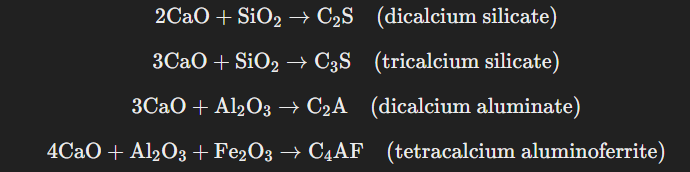
The result of these reactions is a mixture of calcium silicates and aluminates, which fuses into hard clinker stones, usually measuring 5-10mm in size. These clinkers are then cooled rapidly by blowing air into the kiln.
4. Grinding of Clinkers
Once cooled, the clinkers are sent to ball or tube mills for grinding. During this process, a small amount of gypsum (about 2-3%) is added to control the setting time of the cement. The gypsum reacts with tricalcium aluminate to form tricalcium sulfoaluminate, which retards the cement’s initial setting time, preventing it from hardening too quickly.
The final product is a fine powder, which is the Portland cement that will be used in various construction applications.
5. Storage and Packaging
After grinding, the finished cement is stored in large silos. From these silos, cement is packaged in either bulk containers or 50kg bags, ready for distribution. The cement is then transported to various locations for use in construction projects.
Conclusion
The manufacture of Portland cement is a complex process that involves a series of carefully controlled steps, each of which plays a critical role in ensuring the quality and performance of the final product. From the initial mixing of raw materials to the final grinding and packaging, each stage requires precise chemical reactions and careful management. Whether using the dry or wet process, the end result is the same: a strong, versatile material that is the foundation of modern construction. Understanding the chemistry behind cement production not only sheds light on the importance of each step but also highlights the ingenuity behind Joseph Aspdin’s groundbreaking invention nearly two centuries ago.
